
So, that's why we put the lone pair in the same plane as the two atoms comprising the "trigonal" portion of the atom.Īs to the altered degrees of each bond, the bond angles should be a bit smaller due to electron-electron repulsion. H30+ Molecular geometry Valence electrons 5.


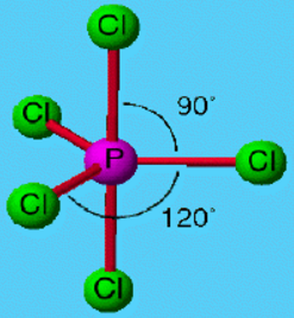
CIF, Molecular Geometry: Bond Angle (s): Lewis Structure: El. In contrast, if we placed the lone pair at the "top" or "bottom" (perpendicular plane) of the molecule, every bond angle would be 90 degrees, which would be much smaller than some of the 120 degree angles possible with the other placement. Using VSEPR, determine the electron pair geometry, the molecular geometry, and the bond angle (in degrees) for each compound. Normally, when all these regions are bonding, the molecule has 120 degree angles between the three atoms making up the 'trigonal' part of the shape and 90 degree angles between the two atoms of the 'bipyramidal. When we place it in the "trigonal" part of the shape, there is an approximately 120 degree angle between the lone pair and other two bonding pairs in the same plane, and a 90 degree angle with the other two perpendicular bonding pairs. For the seesaw shape, we have 5 regions of electron density (trigonal bipyramidal), consisting of 4 bonding pairs and 1 lone pair. When a lone pair is added, we want to place it as far away as possible from the bonding pairs due to electron-electron repulsion. The name 'seesaw' comes from the observation that it looks like a playground seesaw. Normally, when all these regions are bonding, the molecule has 120 degree angles between the three atoms making up the "trigonal" part of the shape and 90 degree angles between the two atoms of the "bipyramidal" part of the shape in relation to the other atoms. Trigonal pyramidal molecules, while still adhering to the VSEPR, have bond angles that are bent out of their expected angles, and they can vary (<109 degrees). Disphenoidal or seesaw (also known as sawhorse ) is a type of molecular geometry where there are four bonds to a central atom with overall C2v molecular symmetry. The F atoms form an octahedron about the central S atom: four of the F atoms form a square with the S atom at the center, and the other two F atoms are above and below the S atom. This approach gives no information about the actual arrangement of atoms in space, however. For the seesaw shape, we have 5 regions of electron density (trigonal bipyramidal), consisting of 4 bonding pairs and 1 lone pair. A lone pair is less restrained than bonding pairs and so takes up more space the bonding pairs (with their atoms) move away from the lone pair in an attempt to. The observed geometry of SF 6, as shown in Figure 7.2, is highly symmetric: all bond lengths are identical and all bond angles are 90o. The Lewis electron-pair approach described previously can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons.


 0 kommentar(er)
0 kommentar(er)
